Stipendiary Lecturer in Neuroscience Dr David A Menassa is one of the senior authors on a new paper in Nature Communications that reports on the use of a novel AI tool which studies cellular interactions in human brain tissues in an automated way. The tool, DeepCellMap, has been co-developed by Dr Menassa with a team of international experts in machine learning and applied mathematics (Mr Theo Perochon & Professor David Holcman, École Normale Supérieure) and advanced spatial statistics (Professor Thibault Lagache, Institut Pasteur).
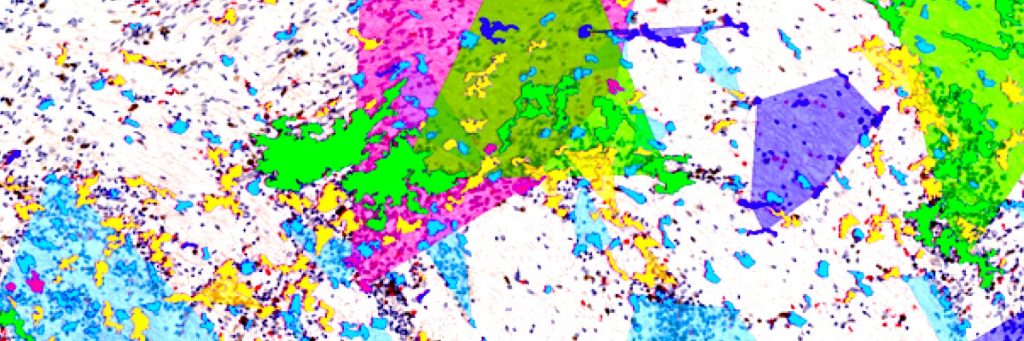
DeepCellMap is an open-source deep learning tool with robust spatial statistics integrated within the same pipeline, allowing the extraction of meaningful metrics of biological and clinical relevance in human tissues. It reveals the shape diversity of microglia during normal human brain development. Microglia are our brain’s immune and phagocytic cells, which participate in brain wiring. These cells are also part of the pathological signature of many neurodegenerative and neurodevelopmental disorders. With DeepCellMap, the team of scientists can identify how microglia cluster as they colonise the brain.
The study aimed to characterise the spatial shape diversity of microglial cells during human brain development and to understand whether blood vessels and microglial cells are linked in fetal cases with brain haemorrhages from mothers who contracted SARS-CoV-2 during pregnancy. The results show that these cells are tightly associated with blood vessels in these cases, suggesting that microglia may respond to, or influence, changes in blood vessel integrity.
This is a rare multidisciplinary effort between artificial intelligence/deep learning, human neurodevelopment, microglial biology, and advanced spatial statistics. DeepCellMap is an adaptable tool that can be deployed on human tissues to elucidate cellular organisation in healthy and pathological conditions, such as neurodevelopmental disorders and neurodegenerative diseases like Alzheimer’s and Parkinson’s.
We do not fully understand the inflammatory cartography of human neurodevelopment and neurodegenerative disease. With DeepCellMap, the elucidation of cellular organisation can be determined in an efficient and thorough manner to reveal patterns that are otherwise not visible to us. This can help us develop targeted hypotheses to unravel detailed mechanisms and develop treatments aimed at targeting inflammation in the brain. Furthermore, DeepCellMap can help us study interactions between cell types in tissues. In diseases like Alzheimer’s, nasal sprays are already being administered to dampen inflammation which underlines the importance of further elucidating how the immune response is orchestrated in other neurodegenerative diseases.
Dr David Menassa said:
DeepCellMap can be applied to any cell type, is highly adaptable and can unravel novel biological patterns in human tissues. Artificial intelligence tools will become the future in neuropathology diagnostic practice and tools like DeepCellMap are just the start of a revolution in expedited and automated histological analyses using deep learning and spatial statistics.
Image: microglial cluster segmentation